Biopharma Services
Your specialty lab partner supporting clinical research
Integrating data from anatomic, cellular, and molecular investigations to provide clinical research teams with actionable information for patient monitoring and treatment decisions
Your specialty lab partner supporting clinical research
Integrating data from anatomic, cellular, and molecular investigations to provide clinical research teams with actionable information for patient monitoring and treatment decisions

What Sets Us Apart
Molecular Pathology Laboratory Network (MPLN) has been a trusted leader in laboratory medicine for over 35 years, with proven expertise in hematopathology, solid tumor oncology, immunology, infectious disease, and women’s health. Since our founding in 1989, we have been at the forefront of applying molecular pathology techniques to both diagnostics and clinical research.
Our integrated approach combines the precision and reliability of our accredited diagnostic laboratory with the agility and innovation of our clinical research division, Geneuity Clinical Research Services. Founded in 2007, Geneuity Clinical Research Services provides specialized support such as project and data management, tailored to the needs of global clinical trials. At MPLN/Geneuity, clients benefit from testing performed in a CAP/CLIA/ISO 15189-accredited facility – paired with the flexibility, global logistics, and responsive service of a world-class clinical research organization.
Our Impact
Improving medicine through innovative science
We offer a unique combination of expertise that can be effectively applied to your clinical trial services.
Supporting more than
Over
Years
of Experience in clinical trial and IVD studies
Clinical trials completed
Our clinical expertise
Hematopathology and solid tumor oncology
Our board-certified hematopathologists and scientists bring global phase I–IV expertise, delivering the speed, precision, and insights needed to move from discovery to approval with confidence.We provide comprehensive diagnostics for blood cancers (e.g., NHL, CLL, AML, MM, CML, MDS) and solid tumors (e.g., lung, prostate, breast, ovarian, bladder). By integrating pathology, molecular testing, cytogenetics, FISH, immunohistochemistry, and immunology, MPLN/Geneuity:
- Accelerates biomarker validation,
- Delivers high-quality, reproducible results,
- Supports clinical trialsto positively impact patient care
At MPLN/Geneuity, we go beyond testing. We partner with our clients to drive innovation in oncology trials.
GI and other specialty oncology
MPLN/Geneuity partners with a global network of specialty pathologists to support a broad range of therapeutic areas. Our pathology experts cover specialty areas such as gastrointestinal, kidney, urology, cytology, and dermatology, with flexibility to expand as clinical trial needs evolve.Through this collaborative model, enhanced by digital pathology for efficiency and consistency, clients gain access to:
- Deep clinical insight across multiple indications,
- Uniform, high-quality reporting through our workflows,
- Streamlined data integration for harmonized clinical trial reports
Trusted by leading pharma and CRO partners worldwide, MPLN/Geneuity combines specialized expertise with consistent reporting to drive global trial success.
Integrated Services
The Power of a Global Network Managed by One Trusted Partner
From advanced specialty laboratory testing to seamless project execution, we deliver centralized, harmonized services to support clinical research around the globe.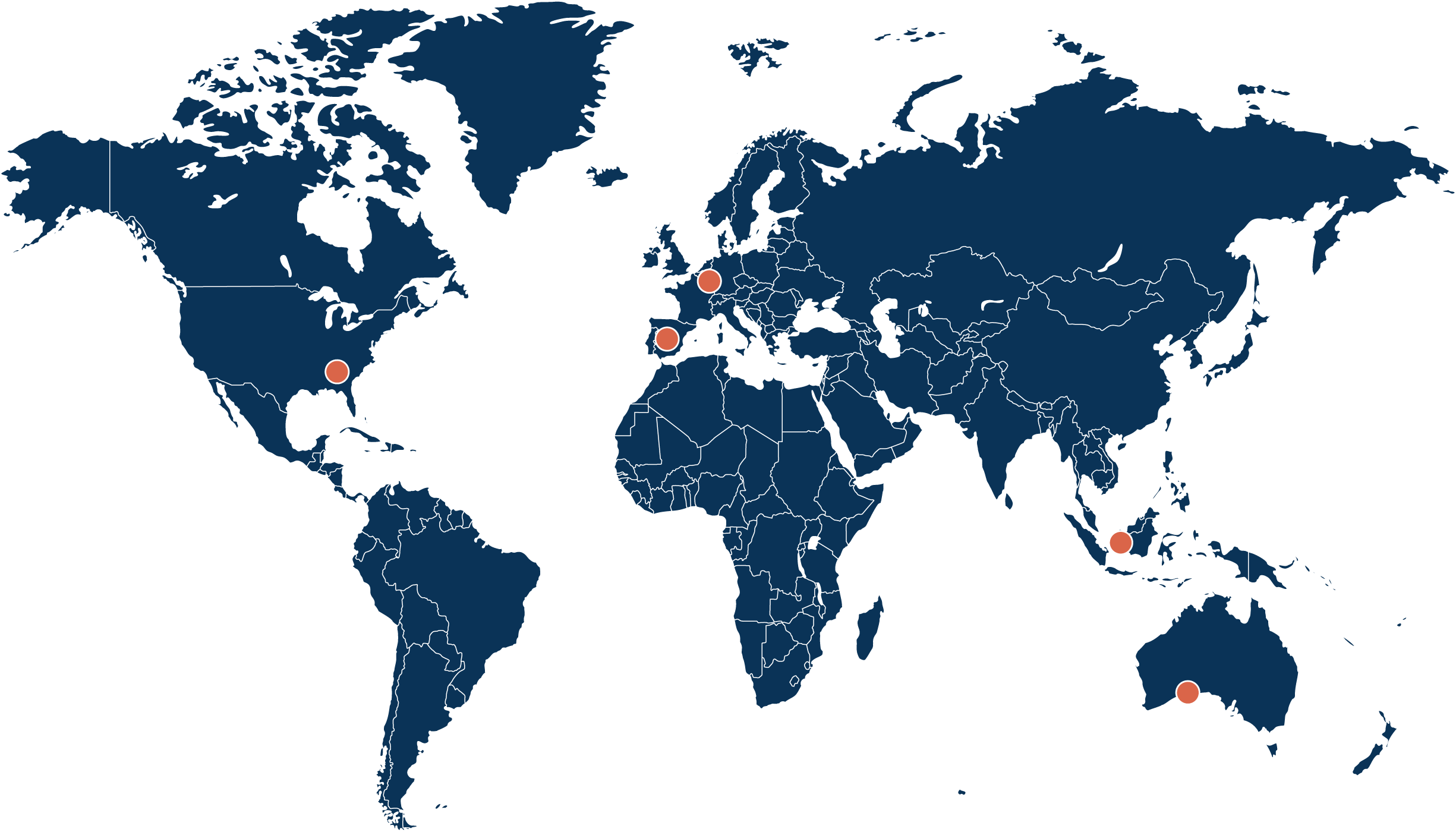
Project and Data Management
Our customer-focused clinical project and data managers provide dedicated support throughout the project lifecycle, keeping you informed and on budget at every important milestone. Our data management systems ensure secure, accessible, and integrated patient information across all services.
Project Management:
Dedicated teams for end-to-end global project oversight.
Data Security:
Top-tier encryption and compliance with global standards.
Integrated Systems:
Streamlined data sharing for seamless access to patient reportables.
Experienced Professionals:
All MPLN/Geneuity project managers have extensive backgrounds in biomedical sciences and years of experience managing global phase 1-4 clinical trials. This expertise gives us a unique competitive advantage, equipping us with a deeper understanding of what it takes to achieve success.
Exceptional Communication:
Our project and data management teams work together seamlessly, ensuring results are delivered on time and within project specifications.Our clients enjoy industry-leading customer service and timely query resolution at every step of the clinical trial lifecycle.
Project Management:
Dedicated teams for end-to-end project oversight.
Data Security:
Top-tier encryption and compliance with global standards.
Integrated Systems:
Streamlined data sharing for seamless access to patient data.
Experienced Professionals:
All MPLN/Geneuity project managers have extensive backgrounds in biomedical sciences and years of experience managing global phase 1-4 clinical trials. This expertise gives us a unique competitive advantage, equipping us with a deeper understanding of what it takes to achieve success.
Exceptional Communication:
Our project and data management teams work together seamlessly, ensuring results are delivered on time and within project specifications.Our clients enjoy industry-leading customer service and timely query resolution at every step of the clinical trial lifecycle.








