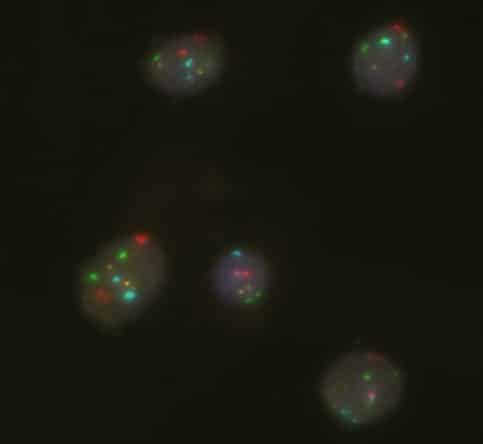
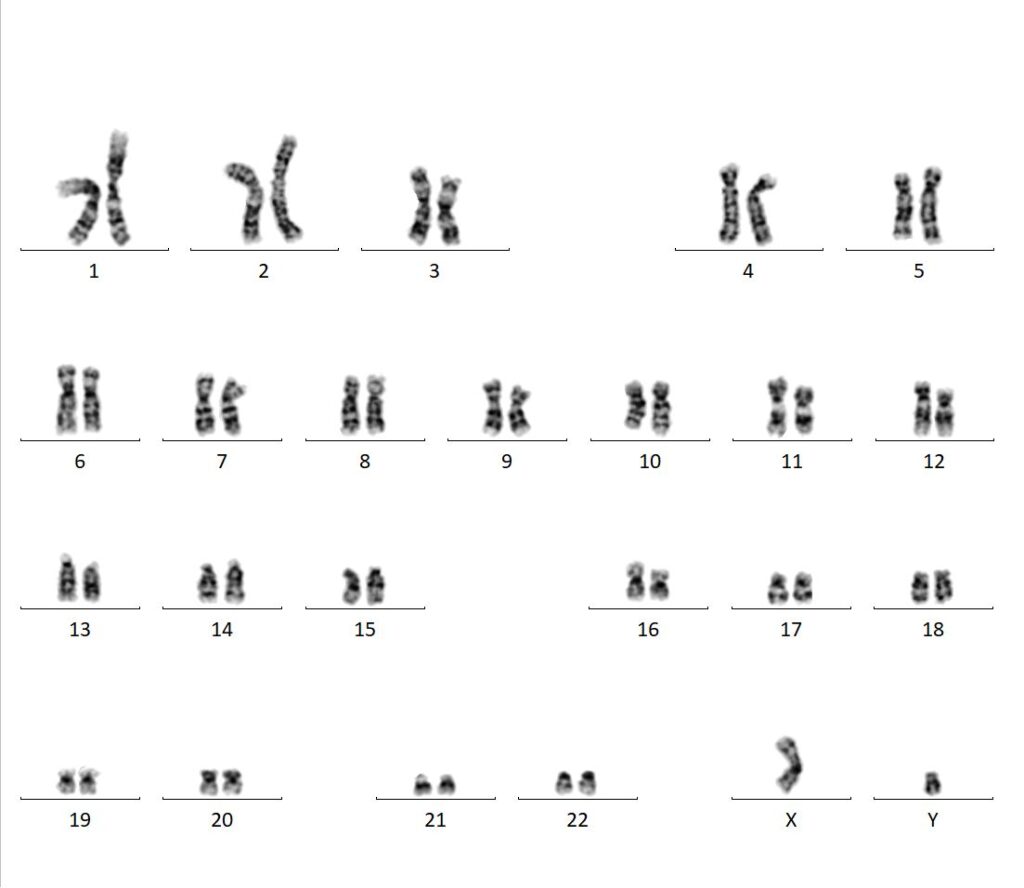
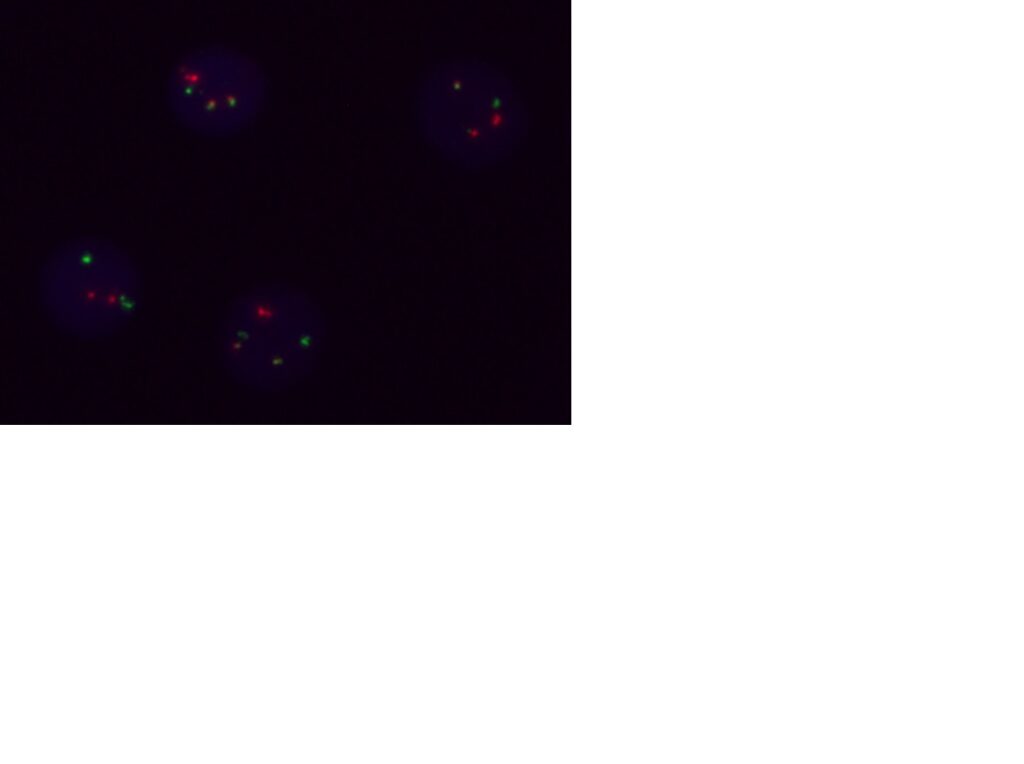
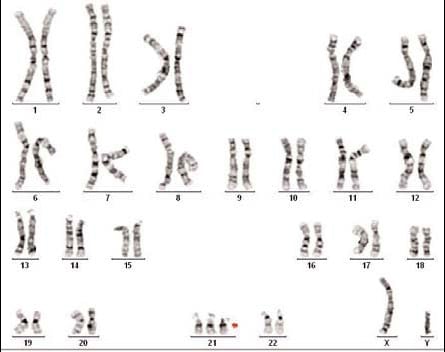
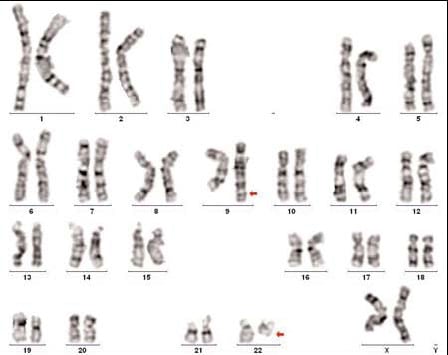
Molecular Pathology Laboratory Network, Inc. (MPLN) has been on the forefront of laboratory medicine since its inception. MPLN Cytogenomics provides a broad menu of testing, including both chromosome analysis and fluorescent in situ hybridization (FISH) with industry-leading turn-around times and global harmonious coverage for traditional cytogenetics and specific FISH chromosomal analyses. Board-certified laboratory directors work closely with licensed cytogenetic technologists to ensure accurate and efficient testing, competitive pricing, and the highest quality of service. Our expert team includes individuals with decades of experience in this specialized field.