Cytogenomics
Home » Cytogenomics
Cytogenomics
FISH
MPLN offers an extensive FISH menu with over 40 loci and multiple disease-specific panels capable of detecting gene amplification/deletions, translocations, and inversions. Testing is performed on bone marrow, blood, and FFPE samples with the following offerings:
- >40 loci targeted by FISH probes
- Plasma cell enrichment for the detection of plasma cell disorders
- Stat turn-around on suspected acute promyelocytic leukemia cases (PML::RARA)
- Stat turn-around on acute myeloid leukemia cases
- Urine analysis for bladder cancer testing
- Custom FISH assay or FISH panel development
Innovation in Cytogenomics
Our dedicated scientific experts are at the forefront of diagnostic technology in their field and actively pursue novel solutions. Read about recent publications from our cytogenetics director and our comprehensive testing approach to Bladder Cancer.

Constitutional Chromosome Studies
Cytogenetic analysis to determine if constitutional abnormalities are present is performed for a variety of indications including multiple congenital abnormalities, mental retardation of unknown etiology, abnormalities of growth, features of a recognized genetic syndrome, recurrent pregnancy loss, prenatal diagnosis via amniocentesis, mosaicism, stillbirth, fetal loss, or molar pregnancy. Adjunct studies such as FISH or other molecular and biochemical testing can be performed in addition to chromosomal analysis.
Chromosome Analysis (Traditional Cytogenetics)
Chromosomal abnormalities are a major contributor to disease. Cytogenetic analysis is critical in the diagnosis, prognosis, and treatment selection of numerous hematopoietic disorders, including leukemia and lymphoma. In addition to oncology, chromosome disorders are responsible for a large proportion of individuals with recurrent pregnancy loss, infertility, intellectual disability, and congenital malformations. Our test menu includes offerings covering both oncology and constitutional chromosomal analysis.
Oncology chromosome analysis
This test is intended for diagnosing, prognosis, and monitoring hematopoietic neoplasms.
- Bone marrow aspirate
- Bone marrow core
- Peripheral blood
- Tissues for short-term culture (lymph node, fine needle aspirate, cerebral spinal fluid, vitreous fluid)
Constitutional chromosome analysis
This test is intended for postnatal constitutional studies. It can be used to diagnose aneuploid syndrome or detect a chromosome translocation.
- Peripheral blood
- Newborn blood

Cancer Chromosome Studies
Cytogenetic analysis in neoplastic diseases involves the study of the cancer cells themselves. In leukemia, a bone marrow aspirate is usually obtained for study. In some cases, peripheral blood is used in place of the bone marrow, particularly if the white blood cell count is >10,000. The purpose of the cytogenetic study in hematological disorders is to detect the presence of acquired chromosome changes, i.e., those aberrations that have arisen secondary to the disease state. The study of chromosomes in leukemia serves two functions:
- To assist in a more accurate diagnosis, thereby
providing important prognostic information - To identify the sites of consistent rearrangements
and identify common changes early in order to
characterize many clonal lines
Specific chromosome abnormalities often correlate with particular subtypes of disease. Serial samples from the
patient permit the study of cytogenetic patterns during the various stages of a patient’s clinical course.
Constitutional Chromosome Studies
Cytogenetic analysis to determine if constitutional abnormalities are present is performed for a variety of indications including multiple congenital abnormalities, mental retardation of unknown etiology, abnormalities of growth, features of a recognized genetic syndrome, recurrent pregnancy loss, prenatal diagnosis via amniocentesis, mosaicism, stillbirth, fetal loss, or molar pregnancy. Adjunct studies such as FISH or other molecular and biochemical testing can be performed in addition to chromosomal analysis.
FISH
In addition to offering high quality chromosome analysis, the cytogenetics laboratory also specializes in fluorescence in situ hybridization (FISH). FISH, a molecular cytogenetic technique, enables the analysis of disease specific abnormalities. It is offered for the detection of cryptic rearrangements, microdeletion syndromes, aneuploidy, and marker chromosome identification.
One Source
Coordinating laboratory tests and results within one facility, MPLN provides a single source for anatomic pathology, FISH, flow cytometry, cytogenetics, and molecular testing.
Using one source for your laboratory testing
provides simpler logistics for ordering, molecular
reflex testing, reporting, billing, and patient
management.
Contact Us
For more information, visit us online at www.MPLNet.com
or contact us at 800.932.2943.
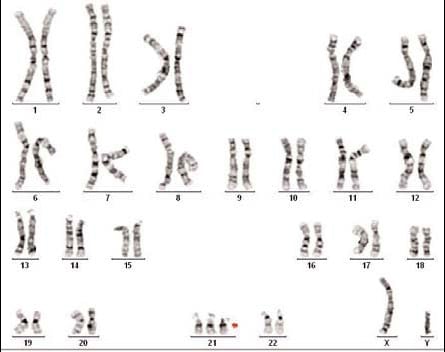
Karyotype showing trisomy 21 consistant with
a clinical diagnosis of Down syndrome
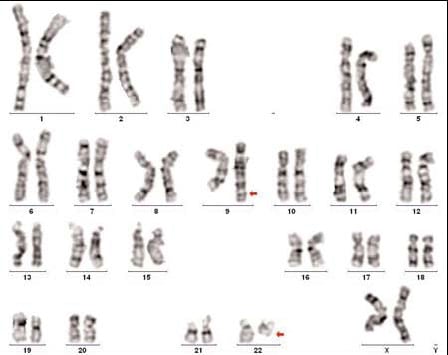
Karyotype showing the Philadelphia
translocation involving chromosomes 9 and 22
| Test Name | Specimen Requirements |
| Cancer cytogenetics (CYTO BM, CYTO UPB, CYTO ST) |
7mL (min. 5mL) whole blood or 3mL (min. 1mL) bone marrow in sodium heparin, 5mm3 bone marrow core biopsy or fresh tissue in transport media, 4mL (min. 2mL) fine needle aspiration in tissue transport media, 15mL ascites, gastric pleural effusions in plain tube |
| Prenatal chromosome analysis (CYTO PN, CYTO AF) |
30mL (min 20mL) amniotic fluid in 2-3 sterile tubes |
| Constitutional tissue chromosome
analysis (CYTO TC) |
Products of conception, fetal tissue, 1cm skin biopsy or other solid tissue in sterile tissue transport media |
| Constitutional peripheral blood
chromosome analysis (CYTO PB) |
7mL (min. 5mL) whole blood or 1ml newborn blood or 2mL (min. 1mL) percutaneous umbilical cord blood in sodium heparin |
| Fluorescence in situ hybridization | Peripheral blood, amniotic fluid, solid tissue, and bone marrow samples with or without routine chromosome studies; please see catalogue for more detailed information |









