For myelodysplastic syndromes, current therapy is selected based on risk, transfusion needs, percent of bone marrow blasts, cytogenetic and mutational profiles, comorbidities, potential for allogeneic stem cell transplantation (alloSCT), and prior exposure to hypomethylating agents (HMA).
Better diagnosis and prognostic stratification may allow a more precise and personalized treatment of MDS with novel agent combinations, leading to improved therapeutic algorithms.
Here at MPLN-Genuity, we have combined our decades of diagnostic expertise and understanding of disease mechanisms with our enhanced global capability to prepare a comprehensive suite of CAP/CLIA and ISO 15189 analytical methods.
This global testing will assist clinical teams and biopharma companies in their drug development projects to implement a refined cytogenetic and molecular assessment of MDS that could improve prognostic stratification and patient monitoring with the integration of clinical, hematological, and genomic data.
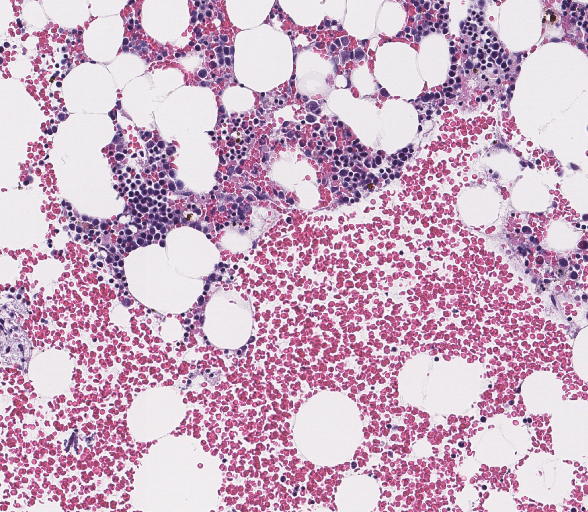
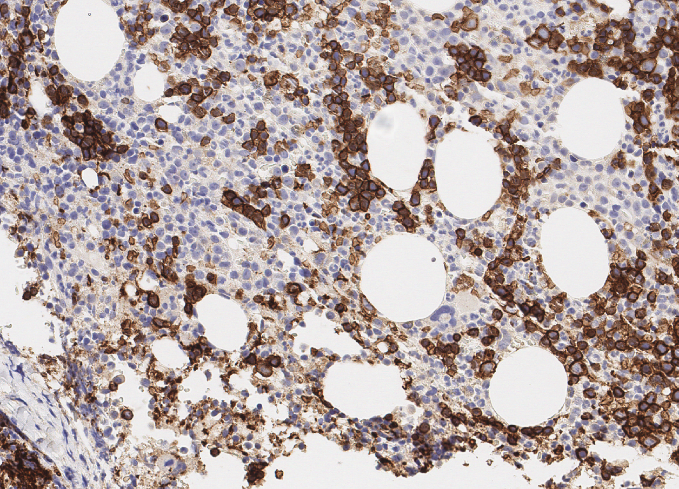
Myelodysplastic Syndrome Analytical Methods
-
- Global Centralized Bone Marrow aspirate assessment by hematopathologist
-
- Pathologist Bone Marrow Evaluation (Aspirate), (BMA) inclusive of Iron and Wright Giemsa stain reads.
-
- Global Centralized Bone Marrow aspirate assessment by hematopathologist
-
- Global Centralized Bone Marrow Biopsy assessment by hematopathologists
-
- Pathologist Bone Marrow Evaluation (BMB)
-
- includes evaluation and reporting of All IHC stains.
-
- Cellularity and megakaryocytic dysplasia.
-
- With inclusive additional IHC workup as requested and agreed with the client
-
- CD34, CD71, CD117, MPO , CD42b
-
- Global Centralized Bone Marrow Biopsy assessment by hematopathologists
-
- Regionalized Complex Karyotyping and reporting.
-
- USA, EMEA, APAC,
-
- A central report issued by MPLN/Geneuity
-
- Globalized DNA extraction and Myeloid Panel NGS
-
- Myeloid Sequencing Panel.
-
- Panel contents vary on discussion with the Client.
-
- Mutations most often seen in MDS cells include those in the DNMT3A, TET2, ASXL1, TP53, RUNX1, SRSF2, and SF3B1
Recent Posts

Molecular Pathology Laboratory Network, Inc. Selects HALO AP® to Deliver Comprehensive Reference Laboratory Services and Biopharma Laboratory Support
Indica Labs announced today that Molecular Pathology Laboratory Network, Inc. (MPLN), a leader in laboratory medicine, hematopathology, and solid tumor oncology testing, has selected the HALO AP® platform from Indica Labs to deliver digital pathology

Data Management at Geneuity
Effective data management is crucial in the fast-paced world of clinical trials. At MPLN, our journey through data management has evolved significantly since our first clinical trial in 2004.

A multicenter analysis of individuals with a 47,XXY/46,XX karyotype
This multicenter analysis examines the genetic and clinical characteristics of individuals with a 47, XXY/46, XX karyotype. Led by an experienced team of researchers, including Tiffany Guess, Ferrin C. Wheeler,









