Biopharma Services
Your specialty lab partner supporting clinical research
Integrating data from anatomic, cellular, and molecular investigations to provide clinical research teams with actionable information for patient monitoring and treatment decisions
Your specialty lab partner supporting clinical research
Integrating data from anatomic, cellular, and molecular investigations to provide clinical research teams with actionable information for patient monitoring and treatment decisions
MPLN’s Commitment to Excellence in Clinical Trials:
Geneuity Clinical Research Services
Clinical trials demand both advanced laboratory capabilities and robust operational support. Through our wholly owned subsidiary, Geneuity Clinical Research Services, MPLN provides specialized Project Management, Data Management, and Commercial Operations services. By combining cutting-edge diagnostic science with proven global trial strategies, MPLN/Geneuity accelerates research, improves patient outcomes, and earns the trust of leading biopharma clients worldwide.

Improving medicine through innovative science
We offer a unique combination of expertise that can be effectively applied to your clinical trial services.
Supporting more than
Over
Years
of experience in clinical trial and IVD studies
Clinical trials completed
Our Solutions
IHC and Multiplex IHC
Precise tissue analysis through immunohistochemistry and multiplex techniques for detailed insights
Genomic Solutions
Advanced RNA, DNA, and liquid biopsy testing for personalized treatment strategies
Cytogenetics & FISH
Comprehensive genetic testing to support targeted therapies and disease management
Project Management & Data Management
Expert project & data managers support every stage of the project lifecycle
Explore our comprehensive range of testing solutions designed to support your clinical trials.
Integrated Services
The Power of a Global Network Managed by One Trusted Partner
From advanced specialty laboratory testing to seamless project execution, we deliver centralized, harmonized services to support clinical research around the globe.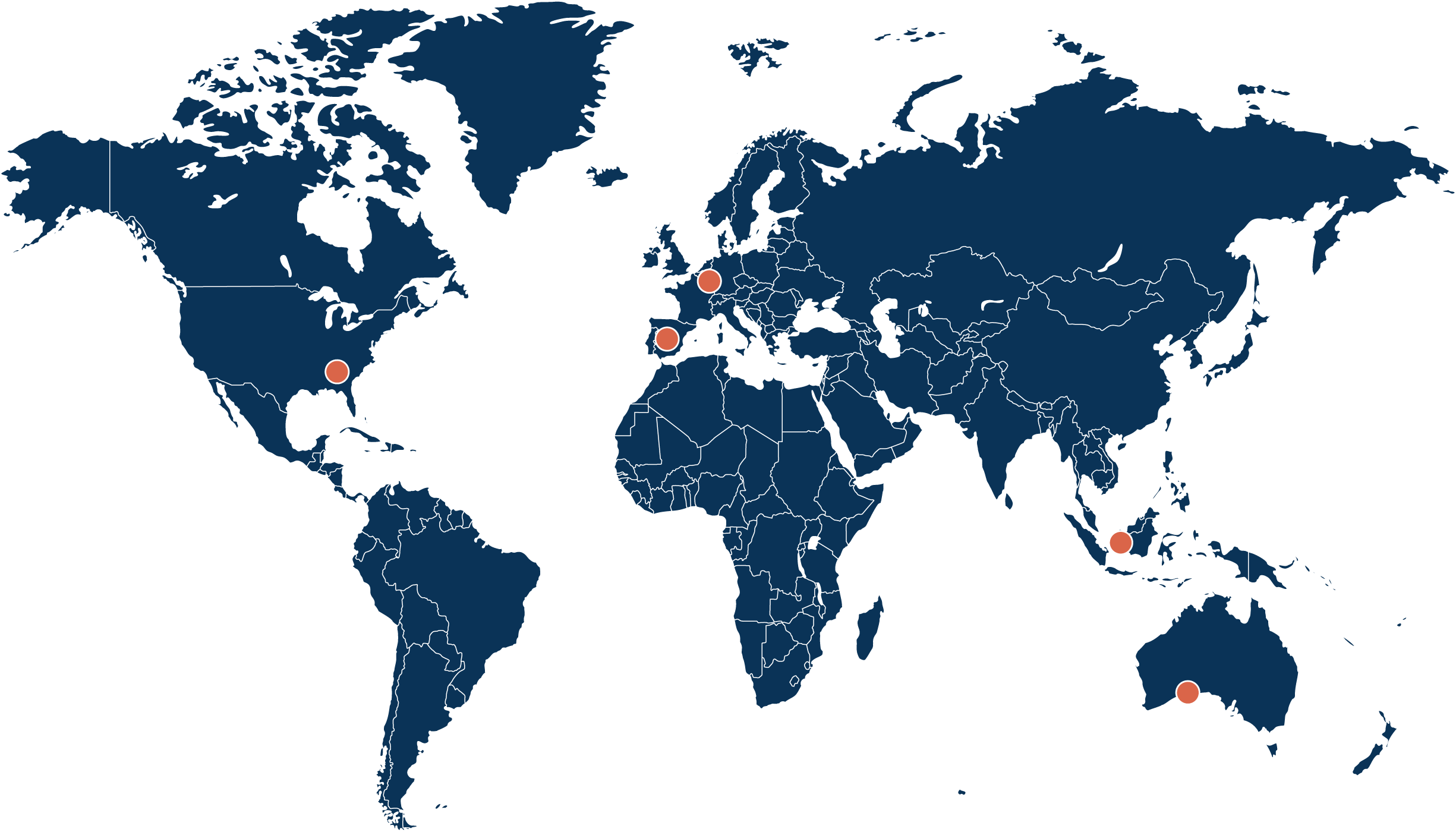
Project and Data Management
Our customer-focused clinical project and data managers provide dedicated support throughout the project lifecycle, keeping you informed and on budget at every important milestone. Our data management systems ensure secure, accessible, and integrated patient information across all services.
Project Management:
Dedicated teams for end-to-end global project oversight.
Data Security:
Top-tier encryption and compliance with global standards.
Integrated Systems:
Streamlined data sharing for seamless access to patient reportables.
Experienced Professionals:
All MPLN/Geneuity project managers have extensive backgrounds in biomedical sciences and years of experience managing global phase 1-4 clinical trials. This expertise gives us a unique competitive advantage, equipping us with a deeper understanding of what it takes to achieve success.
Exceptional Communication:
Our project and data management teams work together seamlessly, ensuring results are delivered on time and within project specifications.Our clients enjoy industry-leading customer service and timely query resolution at every step of the clinical trial lifecycle.
Project Management:
Dedicated teams for end-to-end project oversight.
Data Security:
Top-tier encryption and compliance with global standards.
Integrated Systems:
Streamlined data sharing for seamless access to patient data.
Experienced Professionals:
All MPLN/Geneuity project managers have extensive backgrounds in biomedical sciences and years of experience managing global phase I-IV clinical trials. This expertise gives us a unique competitive advantage, equipping us with a deeper understanding of what it takes to achieve success.
Exceptional Communication:
Our project and data management teams work together seamlessly, ensuring results are delivered on time and within project specifications. Our clients enjoy industry-leading customer service and timely query resolution at every step of the clinical trial lifecycle.














