Our Solutions
Our Solutions
IHC and Multiplex IHC
Precise tissue analysis through immunohistochemistry and multiplex techniques for detailed insights
Digital Pathology
Cutting-edge digital imaging for enhanced diagnostic accuracy and collaboration
Genomic Solutions
Advanced RNA, DNA, and liquid biopsy testing for personalized treatment strategies
Cytogenetics & FISH
Comprehensive genetic testing to support targeted therapies and disease management
Project Management & Data Management
Expert project & data managers support every stage of the project lifecycle
Global Logistics
Integrated specialist logistics, sample management & custom kit building
Explore our comprehensive range of testing solutions designed to support your clinical trials.
Our Solutions

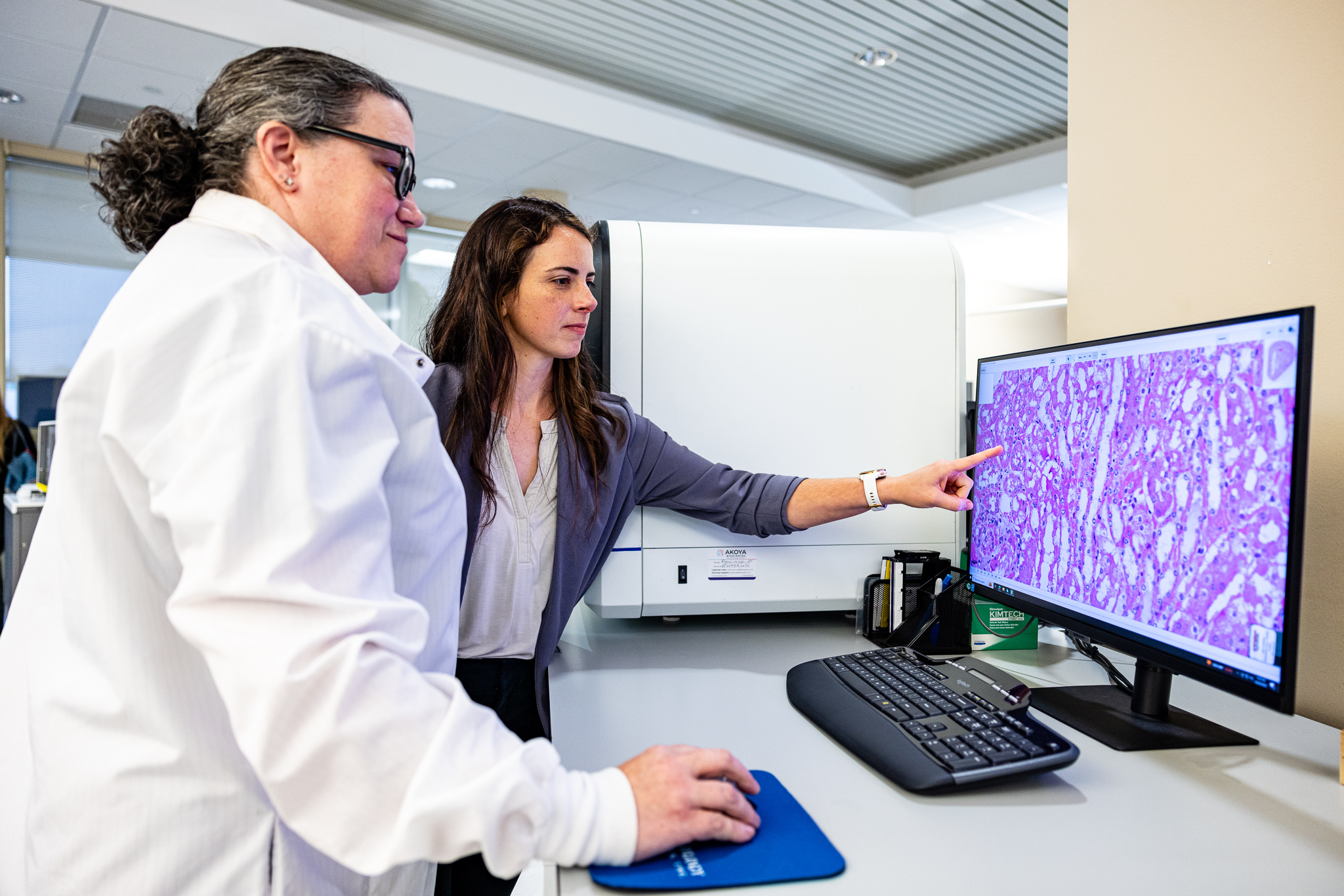
IHC & Multiplex IHC
Leverage our state-of-the-art technology to simultaneously visualize one or more biomarkers within a single tissue section – delivering deeper insights and greater diagnostic precision. With unmatched expertise and advanced capabilities, our team empowers you to characterize complex tissue microenvironments, enabling more effective patient stratification and accurate prediction of treatment responses. Our test menu boasts over 200 fully validated IHC antibodies and offers full-serviceservice tissue processing, including grossing, embedding, cutting, and staining.
Key Features
- Multiplex Capability: Simultaneous detection of multiple biomarkers within the same tissue section for enhanced, multidimensional insights
- Flexible Assay Design: Custom assay development and adaptable testing protocols tailored to your specific research or clinical trial requirements
- Premier Technology: State-of-the-art automated staining equipment from various manufacturers provides consistency and access to popular antibodies
- Expert Support: Comprehensive data analysis and interpretation by our experienced team to ensure reliable, actionable results
Benefits
- Maximized Data from Minimal Samples: Extract more information from limited tissue samples, such as small biopsies (e.g., NSCLC)
- Improved Diagnostics: Achieve more accurate diagnostics and improved patient outcomes through comprehensive biomarker insights
- Enhanced Patient Stratification: Better identify patient subgroups and improve prediction of treatment responses
- Versatile Applications: Whether for biomarker discovery or clinical trial support, our cutting-edge solutions help maximize the value of your tissue samples
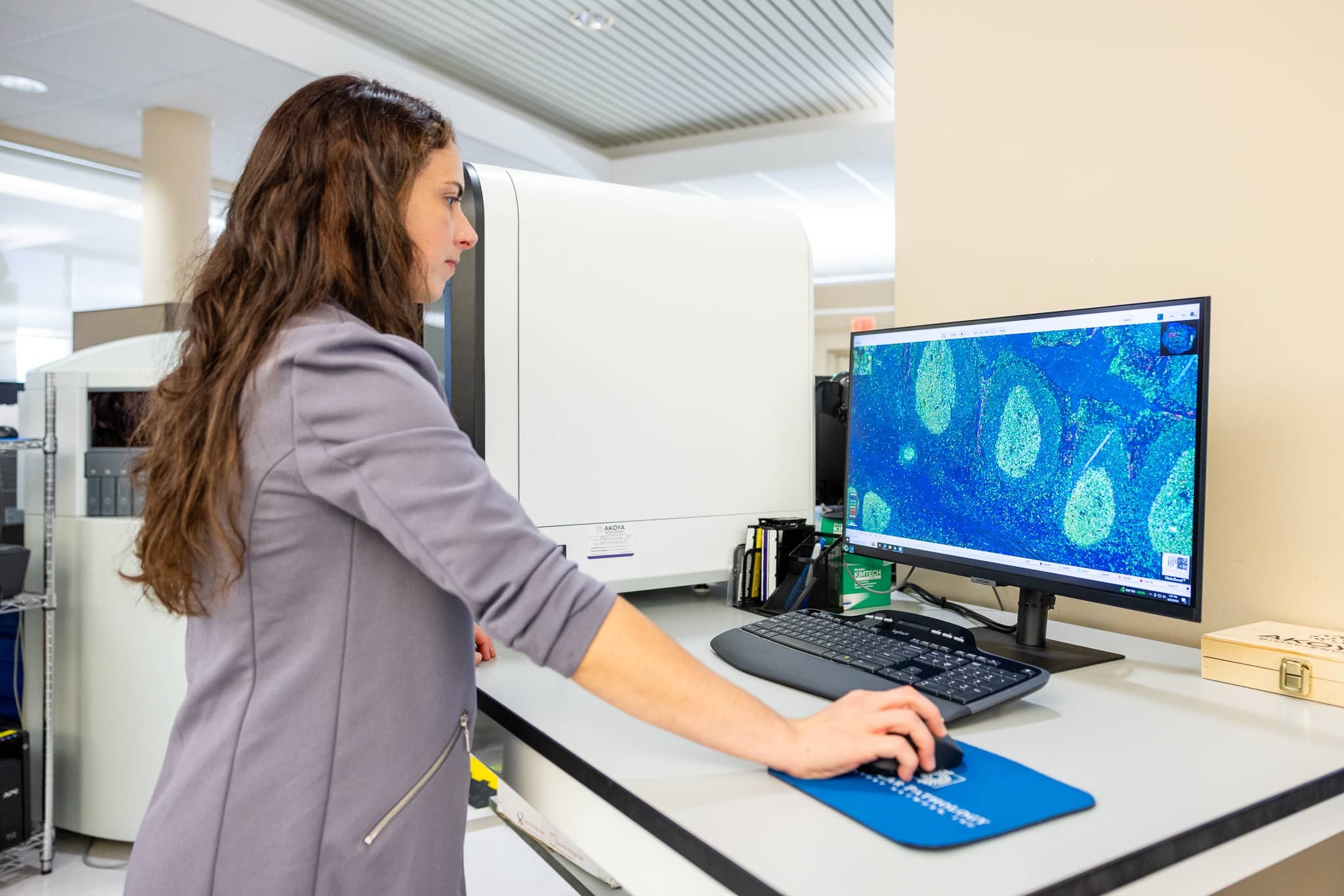
Digital Pathology
Digital pathology is transforming clinical trials by increasing accessibility, reducing costs, enhancing compliance, and streamlining path review. MPLN’s end-to-end digital pathology solutions are designed to optimize clinical research operations and drive improved patient outcomes.
Key Features
- Instant Slide Access: Seamless digital slide retrieval enables real-time access for remote review and analysis, often reducing turnaround times to fit strict screening windows or enhance adjudication workflows
- Cost-Effective Management: Reduces the need for physical slide storage and shipping, simplifying logistics and cutting costs
- Sample Preservation: Digital archiving ensures slides remain intact and accessible without degradation over time
- Long Term Image Archive: Long term digital image storage is available, housed on our secure servers
Benefits
- Remote Collaboration: Enables engagement of expert pathologists worldwide for timely insights and streamlined adjudication
- Faster Decision-Making: Digital sharing accelerates review cycles and fosters more collaborative, informed clinical decisions
- Operational Efficiency: Lowers storage and transport costs, improving trial scalability and efficiency
- Regulatory Readiness: Simplifies regulatory submissions and audit preparation, supporting faster, more accurate FDA packets
- Sample Integrity: Eliminates risks of slide breakage or deterioration, preserving the quality of critical research samples
- Decentralized Trials: Allows for broader patient recruitment and diversity by supporting remote participation across geographically dispersed sites

Genomic Solutions
- DNA Solutions – WGS*, WGMS*, WES*, TSO-Comp, Pillar OncoReveal
- RNA Solutions – Total RNA-Seq*, RNA exome*, mRNA*
- Liquid Biopsy Solutions – TSO-500 ctDNA*, PGDx elio™ plasma complete*
Key Features
- Comprehensive and Regulatory-Backed Panels: Targeted NGS panels, including FDA-approved assays and companion diagnostic designations are available
- Flexible, Tailored Data Analysis: Customizable reporting solutions to meet specific clinical or research needs
- Optimized for Challenging Samples: Robust assay designs support reliable results even from low-yield biopsy material
- Efficient Lab Workflows: Streamlined wet lab processes ensure rapid turnaround times to meet tight screening windows
- Defined Specimen Requirements: Comprehensive online test menu outlines specimen requirements and stability for each assay, enhancing global logistics planning
Benefits
- Comprehensive Detection: Identify variants and key biomarker signatures for a holistic genomic view
- Cost and Time Efficiency: Consolidate multiple biomarker assessments into a single, efficient test with our NGS panel options
- Actionable Results: Delivers clinically relevant insights that are easy to interpret and guide targeted therapy decisions
- Improved Patient Selection: Supports precise patient stratification and recruitment for clinical trials and treatment pathways
- Personalized Treatment Development: Facilitates accelerated translation of genomic data into individualized treatment strategies.
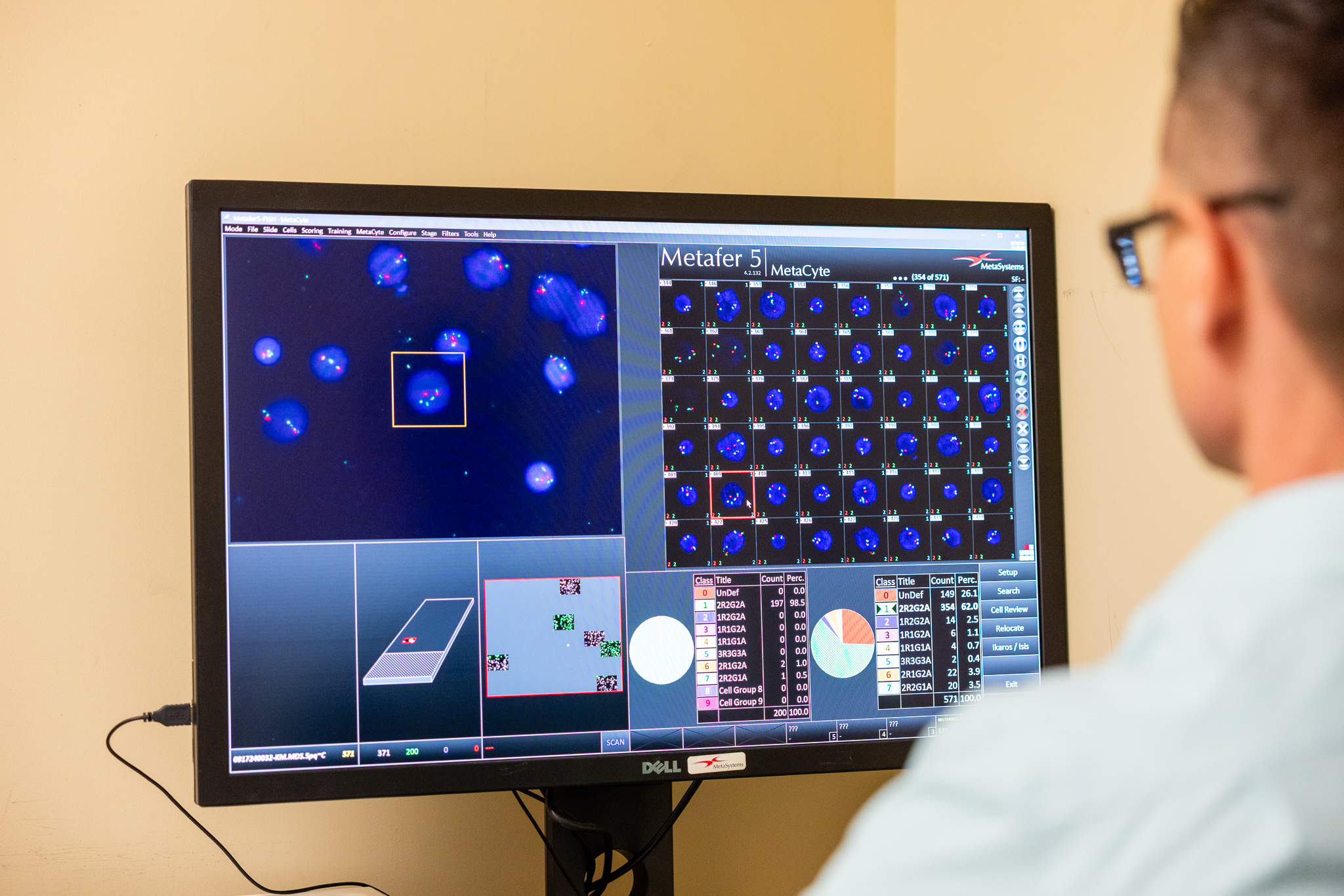
Cytogenetics & FISH
Key Features
- Comprehensive FISH Testing Menu: Access over 40 targeted loci and disease-specific panels to detect gene amplifications, deletions, translocations, and inversions
- Versatile Sample Compatibility: Testing available on bone marrow, peripheral blood, and FFPE tissue samples to support diverse clinical applications and global sample collection
- Specialized Testing Capabilities:
- Plasma cell enrichment for plasma cell disorders
- STAT turnaround for PML::RARA and acute myeloid leukemia (AML)
- Bladder cancer urine analysis
- Custom FISH assay development tailored to specific research or diagnostic needs
- Complete Chromosome Analysis:Karyotyping for oncology, infertility, recurrent pregnancy loss, and congenital disorders
- Global Clinical Trial Support: CLIA/ISO 15189-compliant services available globally to support investigational and clinical study need.
Benefits
- Fast, Actionable Results: Rapid turnaround times enable timely diagnoses and informed treatment decisions
- Trusted Diagnostic Accuracy: Testing led by board-certified experts ensures reliable and clinically validated results
- Tailored Testing Solutions: Customized panels meet specific clinical or research requirements with precision
- Improved Patient Care: Accelerated diagnostics support earlier intervention and optimized treatment planning

Peripheral Blood Mononuclear Cell
We specialize in isolating Peripheral Blood Mononuclear Cells (PBMCs) using reliable techniques that deliver high yields and purity. Whether diving into immunology, exploring cancer therapies, or developing vaccines, our state-of-the-art facility and expert team ensure you receive the best possible service to support your research and clinical trial applications.
Key Features
- High Yield and Purity: Optimized protocols guarantee high yields and purity of PBMCs from peripheral blood
- External Quality Assurance: MPLN participates in external quality assurance programs to maintain qualification status
- Stringent Qualification and Competency: Operators undergo stringent training and qualification by several top ten biopharma companies, assessing criteria for viability, recovery, and functional performance
- Processing Insights: Critical data are captured throughout the isolation procedure and reported to provide key insights into recovery and viability
- Customizable Solutions: We tailor our services to meet your needs - processing is supported along with validation of study-specific PBMC protocols prior to study start
- Cryopreservation Capabilities: Cryopreservation services available to maintain cell viability for long-term storage and future analyses.
Benefits
- Reliable Results: High-quality PBMCs suitable for downstream applications such as flow cytometry, molecular assays, and functional studies
- Efficiency: Streamlined processes lead to reduced turnaround times, facilitating timely research decisions
- Workflow Customization: Ability to tailor services to meet specific research or clinical trial requirements, including custom isolation protocols
- Compliance and Accreditation: Services are conducted in compliance with regulatory standards, ensuring data credibility for clinical trials and research studies